A. What Are Catheters and Tubing Components?
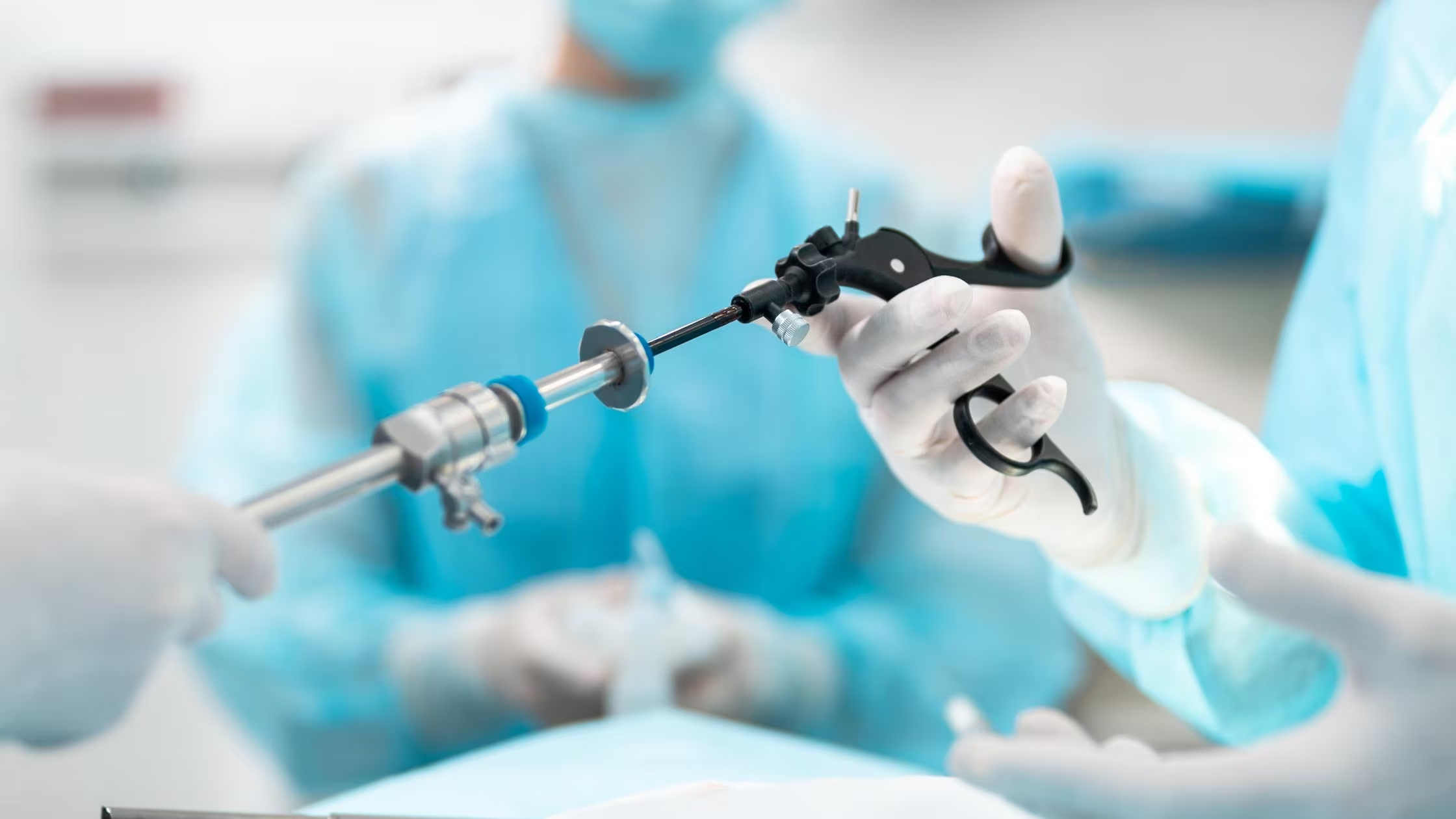
Catheters and tubing components are precision-engineered medical devices designed for minimally invasive access to the vascular system, body cavities, and organs for diagnostic or therapeutic purposes. These critical components include catheter hubs and handles, luer lock and luer slip connectors, Y-connectors and manifolds, guidewire components and tips, introducer sheath valves, hemostasis valves, stopcock assemblies, tubing adapters and fittings, and balloon inflation devices. Used in interventional cardiology, radiology, urology, neurology, and surgical procedures, these components must provide secure connections, smooth fluid flow, atraumatic vessel navigation, and reliable sealing. Precision CNC machining delivers the dimensional accuracy, internal surface smoothness, and tight tolerances required for leak-free performance, minimal pressure drop, and safe patient outcomes during critical procedures.
B. Principais requisitos técnicos
Catheters and tubing CNC machining demands micro-tolerances of ±0.0002″ to ±0.0005″ to ensure proper luer taper fit (6% taper per ISO 594), secure sealing interfaces, and precise internal diameter control for fluid flow calculations. Material specifications require biocompatible, corrosion-resistant materials that withstand sterilization cycles and chemical exposure—typically 316L stainless steel for reusable components, PEEK for structural strength and chemical resistance, or medical-grade plastics for single-use disposable catheters. Surface finish requirements are exceptionally stringent, with Ra 4-16 microinch for internal lumens minimizing turbulence, thrombosis risk, and pressure drop, while external surfaces require Ra 8-32 microinch for smooth tissue contact and atraumatic insertion.
Components must maintain dimensional stability during autoclaving (121-134°C) or ethylene oxide sterilization, provide leak-free sealing under physiological pressures (up to 1200 psi for angioplasty), and demonstrate zero particulate generation that could cause embolic complications. Thread accuracy for luer connections must meet ISO 594-1/594-2 standards with proper engagement and torque resistance. Wall thickness uniformity (±0.0005″), concentricity of internal bores (±0.001″ TIR), and smooth transitions between diameters prevent flow disruption, stress concentrations, and catheter kinking during navigation through tortuous anatomy.
C. Desafios e soluções para o fabrico
Machining catheter and tubing components presents significant challenges including producing micro-scale features without burrs that could cause thrombosis, achieving ultra-smooth internal surfaces in deep small-diameter bores, and maintaining thin-wall integrity without deflection or chatter. Luer taper accuracy requires precise angular control (±0.1°) over short distances, while valve components demand leak-free sealing surfaces with flatness within 0.0002″. Threading micro-components without tool breakage and maintaining concentricity on slender guidewire tips require specialized micro-machining strategies.
Yicen Precision overcomes these manufacturing challenges through ultra-precision 5-axis CNC machining with high-speed spindles (up to 40,000 RPM) for micro-tooling, specialized carbide and diamond-coated tools for superior surface finishes, and advanced CAM programming with optimized chip evacuation strategies for deep internal features. Our Swiss-style CNC lathes with guide bushings provide exceptional support for slender components, minimizing deflection during machining operations. For internal surface finishing, we employ precision honing, electropolishing, and specialized polishing techniques achieving Ra 4-8 microinch in complex geometries.
Quality control measures include coordinate measuring machine (CMM) inspection with micro-touch probes, optical measurement systems for luer taper verification, surface roughness analysis using stylus profilometry, and functional testing including leak testing under pressure, flow rate verification, and connection torque testing. We perform dimensional validation on thread gauges per ISO 594 standards and conduct visual inspection under magnification for burr detection. Material certifications include biocompatibility documentation (ISO 10993, USP Class VI) and chemical composition verification. Learn more about our micro-machining capabilities for your catheter component requirements.
D. Aplicações e casos de utilização
Precision-machined catheter and tubing components enable life-saving minimally invasive procedures across multiple medical specialties:
- Cardiovascular Interventions: Angioplasty catheter hubs, guide catheter connectors, PTCA balloon inflation devices, and hemostasis valve assemblies
- Diagnostic Catheters: Pressure monitoring line connectors, contrast injection manifolds, thermodilution catheter components, and diagnostic guide catheter hubs
- Vascular Access: Central venous catheter hubs, introducer sheath valves, arterial line connectors, and dialysis catheter components
- Urological Devices: Foley catheter connectors, drainage bag adapters, ureteroscopy instrument channels, and cystoscopy irrigation fittings
- Neurological Catheters: External ventricular drain connectors, intracranial pressure monitoring fittings, and spinal catheter adapters
- Surgical Drainage: Chest tube connectors, surgical drain adapters, wound drainage fittings, and suction catheter components
- Drug Delivery Systems: Infusion catheter hubs, port access needles, epidural catheter connectors, and programmable pump tubing fittings
E. Why Choose Yicen Precision for Catheters and Tubing Components?
Yicen Precision delivers specialized expertise in catheters and tubing CNC machining with ISO 13485 certification and comprehensive understanding of ISO 594 luer connector standards and FDA catheter regulations. Our rapid prototyping services provide functional catheter components in 5-7 days, enabling accelerated design validation, flow testing, and clinical evaluation preparation. We offer scalable production from R&D prototype quantities through validated high-volume manufacturing with statistical process control ensuring dimensional consistency critical for catheter system performance.
Our engineering team provides Design for Manufacturability (DFM) consultation optimized for catheter functionality, including fluid dynamics analysis for lumen design, material selection guidance based on biocompatibility and sterilization requirements, and connection interface optimization for secure, leak-free assembly. We coordinate value-added services including electropolishing for ultra-smooth internal surfaces and enhanced corrosion resistance, laser marking for traceability and regulatory labeling, ultrasonic cleaning for particulate-free delivery, and sterilization validation support.
Complete material traceability includes biocompatibility certifications (ISO 10993 testing documentation, USP Class VI validation), material test reports with chemical composition analysis, dimensional inspection reports with luer taper verification data, and certificates of conformance supporting regulatory submissions. We maintain cost-effective catheters and tubing components manufacturing through optimized micro-machining strategies, efficient setup procedures for small components, and streamlined inspection protocols—delivering precision catheter components that ensure patient safety and procedural success without premium pricing. Request your detailed technical consultation and manufacturing quote for precision catheter and tubing components today.