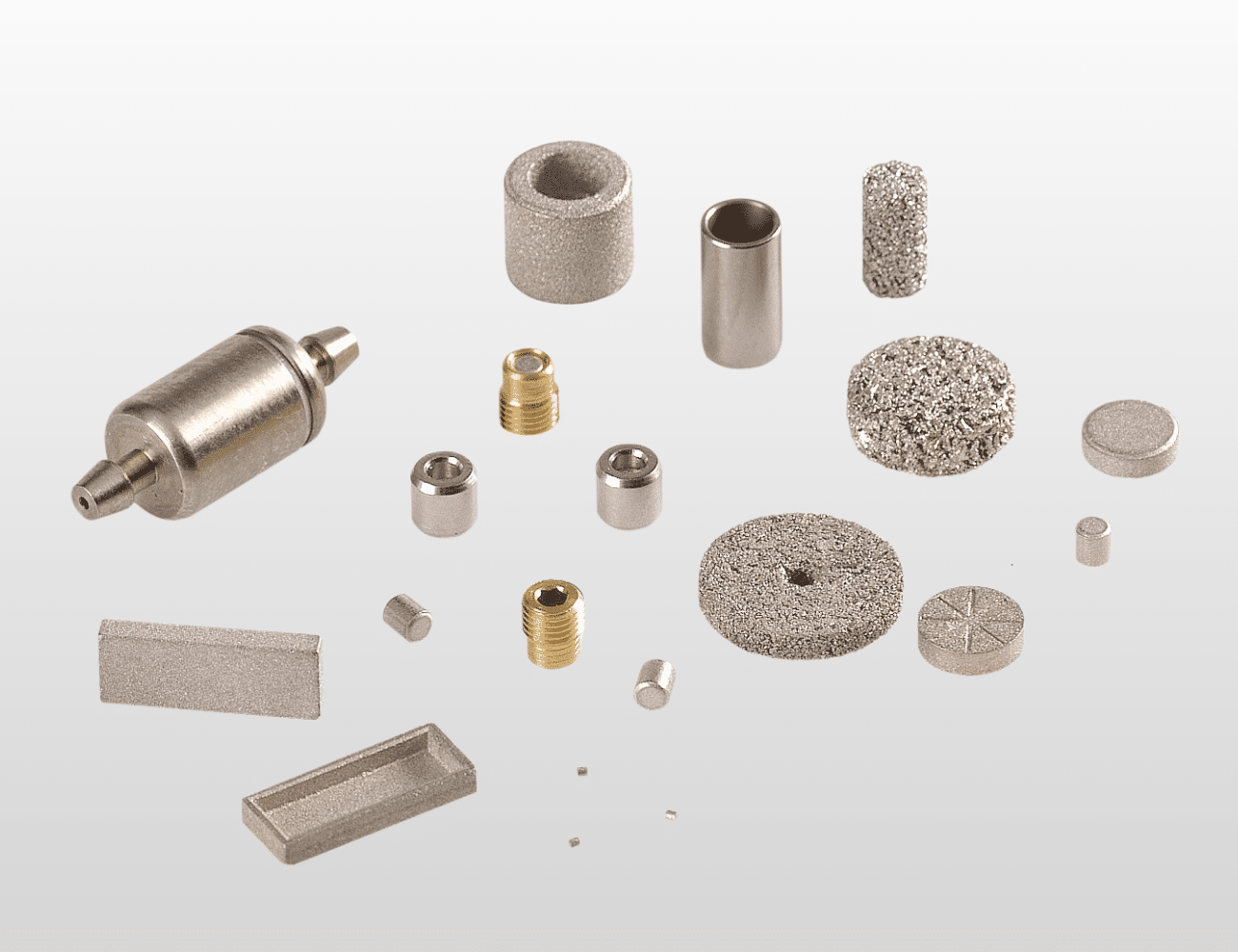
A. What Are Drug Delivery System Components?
Drug delivery system components are precision-engineered medical devices designed to safely and accurately administer pharmaceuticals, biologics, and therapeutic agents to patients through various routes including injection, infusion, inhalation, or transdermal absorption. These critical components include auto-injector mechanisms and housings, insulin pump reservoirs and plunger systems, prefilled syringe barrels and needle shields, infusion pump valve assemblies and flow regulators, pen injector cartridge holders and dose selectors, inhaler actuators and metering valves, transdermal patch applicators, implantable pump reservoirs and refill ports, and needle-free injection systems. Used across hospitals, home healthcare, diabetes management, emergency medicine, and chronic disease treatment, drug delivery components must provide precise dosing accuracy (typically ±5% or better), maintain drug product integrity without contamination or leaching, ensure reliable mechanical function across thousands of cycles, and demonstrate biocompatibility for patient contact surfaces. Precision CNC machining delivers the dimensional accuracy, surface quality, and material compatibility required for regulatory compliance, consistent therapeutic outcomes, and patient safety.
B. Principais requisitos técnicos
Drug delivery system components CNC machining demands micro-tolerances of ±0.0002″ to ±0.0005″ to ensure precise volumetric dosing, leak-free sealing interfaces, and accurate mechanical actuation. Material specifications require certified biocompatible materials with complete chemical inertness—316L stainless steel for reusable injection components, PEEK for chemical resistance to aggressive drug formulations, or cyclic olefin copolymer (COC) and cyclic olefin polymer (COP) for pharmaceutical-grade plastic components. Surface finish requirements are exceptionally stringent, with Ra 4-16 microinch for drug-contact surfaces minimizing protein adsorption and particulate generation, while sealing surfaces require Ra 8-16 microinch with precise flatness (±0.0002″) for reliable o-ring or elastomer seal performance.
Components must maintain dimensional stability during sterilization cycles (steam autoclaving, gamma irradiation, or ethylene oxide), withstand repeated mechanical actuation without wear or dimensional change (10,000+ cycles for reusable devices), and demonstrate zero extractables or leachables that could compromise drug stability or patient safety per ISO 10993-12/18. Internal bore tolerances for syringe barrels and pump cylinders require ±0.0003″ diameter control ensuring consistent volumetric accuracy. Thread precision for luer connections must meet ISO 594 standards, while dose metering mechanisms demand positional accuracy within ±0.5% of target volume. Surface treatments must be validated for compatibility with specific drug formulations including pH extremes, organic solvents, and protein-based biologics.
C. Desafios e soluções para o fabrico
Machining drug delivery system components presents extraordinary challenges including achieving pharmaceutical-grade surface finishes without subsurface damage or contamination, maintaining micro-tolerances on features directly affecting dose accuracy, and ensuring absolute cleanliness preventing particulate contamination that could cause injection site reactions. Thin-walled syringe barrels require uniform wall thickness (±0.0005″) without deflection, while complex valve assemblies with multiple sealing surfaces demand precise surface flatness and parallelism. Threading micro-components, machining deep small-diameter bores with consistent surface finish, and producing features like luer tapers meeting ISO 594 standards require specialized micro-machining capabilities.
Yicen Precision overcomes these manufacturing challenges through ultra-precision 5-axis CNC machining in climate-controlled environments (±0.5°C) with ISO Class 7 cleanroom-adjacent facilities, high-speed spindles (up to 40,000 RPM) with precision micro-tooling for fine features, and advanced CAM programming with optimized parameters for pharmaceutical-grade materials. Our Swiss-style CNC lathes with guide bushing support enable production of long slender components like plunger rods with minimal deflection. For pharmaceutical-grade surface finishes, we employ diamond tooling, precision honing, and electropolishing achieving Ra 4-8 microinch without subsurface defects.
Quality control measures include coordinate measuring machine (CMM) inspection with micro-touch probes and temperature compensation, optical measurement systems for dimensional verification, surface roughness analysis using stylus profilometry, and volumetric accuracy testing for dose-critical components. We perform functional testing including leak testing under pressure, actuation force measurement, and dose delivery verification. Material certifications include biocompatibility documentation (ISO 10993, USP Class VI), extractables/leachables testing data, and chemical composition verification. Our validated cleaning processes with particulate monitoring ensure pharmaceutical-grade cleanliness. Contact us for specialized consultation on your drug delivery component manufacturing requirements.
D. Aplicações e casos de utilização
Precision-machined drug delivery system components enable accurate therapeutic administration across multiple healthcare settings:
- Auto-Injectors: Mechanism housings, plunger rods, needle shields, dose buttons, spring guides, and locking assemblies for epinephrine, biologics, and self-injection devices
- Insulin Delivery Systems: Pump reservoirs, plunger assemblies, cartridge holders, infusion set connectors, dose selectors, and pen injector components
- Infusion Pumps: Valve bodies, flow regulators, pump chambers, check valves, pressure sensors housings, and fluid pathway manifolds
- Prefilled Syringes: Glass or plastic syringe barrels, plunger stoppers, finger flanges, luer lock/slip tips, and needle hub assemblies
- Inhalation Devices: Metered dose inhaler actuators, dry powder inhaler bodies, dose counters, airflow regulators, and nebulizer components
- Transdermal Systems: Patch applicators, microneedle arrays, drug reservoir housings, and controlled-release mechanism components
- Implantable Drug Pumps: Titanium pump housings, refill port assemblies, catheter connectors, reservoir chambers, and programmable valve components
E. Why Choose Yicen Precision for Drug Delivery System Components?
Yicen Precision delivers specialized expertise in drug delivery system components CNC machining with ISO 13485 certification and deep understanding of FDA combination product regulations (21 CFR Part 4) and pharmaceutical compatibility requirements. Our rapid prototyping services provide functional drug delivery components in 5-7 days, enabling accelerated design validation, dose accuracy testing, and human factors evaluation for usability studies. We offer scalable production from R&D quantities for clinical trials through validated commercial-scale manufacturing with statistical process control ensuring dosing accuracy and consistency.
Our engineering team provides Design for Manufacturability (DFM) consultation optimized for drug delivery performance, including volumetric accuracy analysis and tolerance stack-up studies, material selection guidance based on drug formulation compatibility and sterilization methods, and seal design optimization for leak-free performance across shelf life. We coordinate critical value-added services including pharmaceutical-grade electropolishing for ultra-smooth drug-contact surfaces, passivation for corrosion resistance and reduced metal ion leaching, laser marking for traceability without compromising surface integrity, validated ultrasonic cleaning with particulate monitoring, and cleanroom packaging meeting pharmaceutical cleanliness standards.
Complete material traceability includes biocompatibility certifications (ISO 10993 testing documentation, USP Class VI validation), extractables and leachables study support per ISO 10993-12/18, material test reports with chemical composition analysis, dimensional inspection reports with dose-critical feature verification, and certificates of conformance supporting regulatory submissions. We provide process validation documentation (IQ/OQ/PQ protocols), design history file (DHF) support, and maintain batch records for pharmaceutical device requirements. Our cost-effective drug delivery system components manufacturing employs optimized micro-machining strategies, efficient quality control procedures, and streamlined production workflows—delivering life-critical components ensuring therapeutic accuracy and patient safety without premium pricing. Request your detailed technical consultation and manufacturing quote for precision drug delivery system components today.